What Is the Best PH for Your Hydroponic System and How to Maintain it?

The historic concept of hydroponics also has a scientific angle to it. The hydroponics system includes customising various nutrients to fulfil the needs of a particular plant that one chooses to grow.
As we may know, the soil is not used when going for the hydroponics system of planting. It is an area that is expanding and has yet to hit its best extent. The use of hydroponic system extends the potential to gardens in small spaces where insufficient land is accessible.
This method is also used where the soil is not favourable to propagation in arid or barren regions.
While there are multiple and well-established positives of gardening by hydroponics system, the commercial hydroponics industry is yet to enter the forefront. One of the reasons for the same is governments all over the world being sceptical and monitoring businesses related to hydroponics. This is because of its alleged association with the spread of marijuana growing.
Because of environmental issues and a decrease in arable land, the understanding of hydroponics system would eventually be encouraged. The soil-less planting technique is useful for both farmers and home vegetable gardeners as well.
What the Water Wants
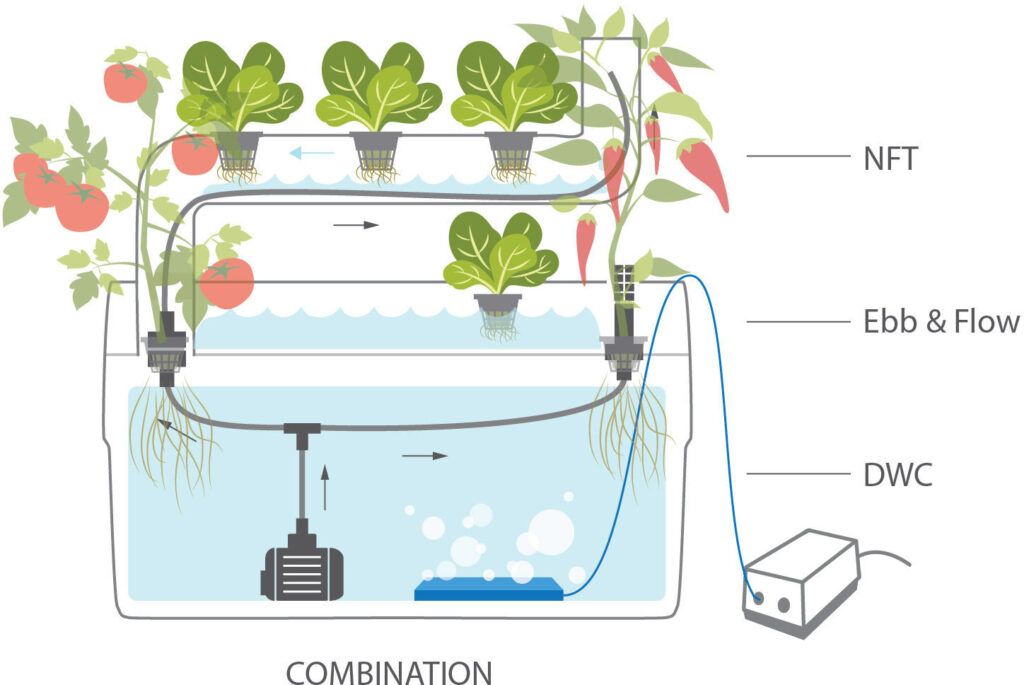
It is of utmost importance to give a conducive and nutritive environment to the grown plants. By taking notice of various nutrients a plant needs, one can make sure to provide them.
Nutrients like calcium, iron, PHosPHorus, manganese etc., naturally occur in soil. But to include them while farming hydroponically is a task nobody should allow sliding.
While making sure other sets of nutrients are well provided to the plants, individuals tend to forget one important thing – the PH. Rather, maintaining the PH.
PH Simplified

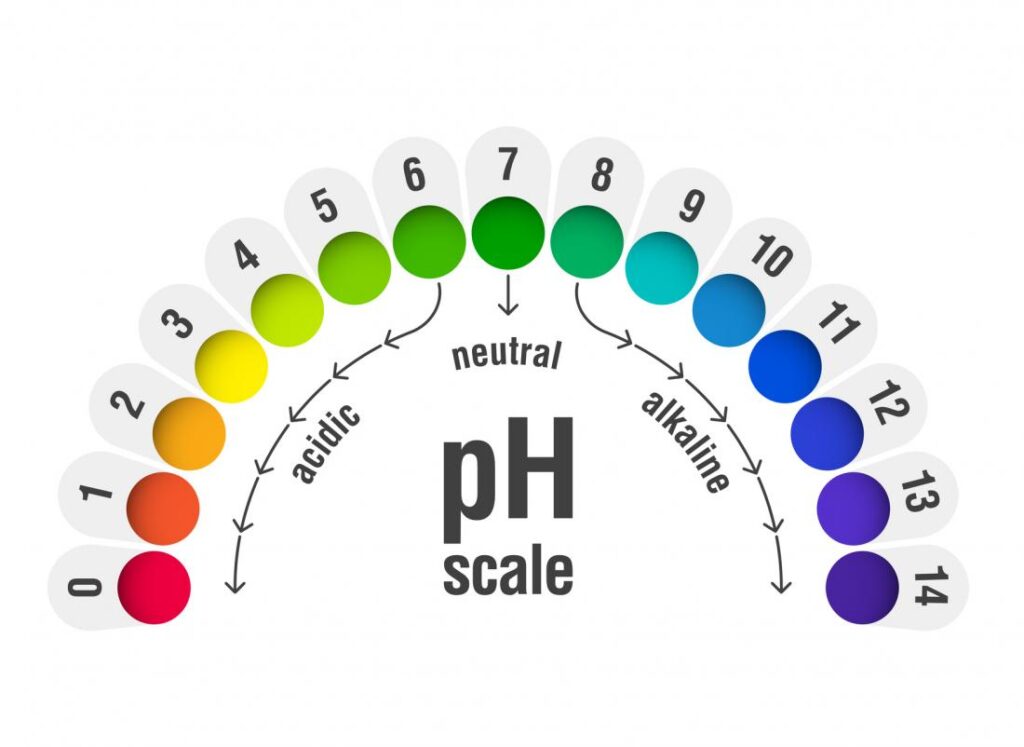
In simple words, PH stands for the level of acidity or alkalinity of any given solutions. It is a measure of the hydronium ion H3O+. The PH of any given substance is measured on a scale of 0 to 14.
Substances can be differentiated whether they are acidic or alkaline. Lower the PH, higher the acidity and vice versa. The middle value, which is ‘7’, is the PH of the water. Substances with a value of 7 are known as neutral.
The ideal PH of a plant grown by hydroponics system is different from those grown in soil. For a majority of crops, ranging from 5.5 to 6.5 is the most appropriate. However, people practising commercial farming narrow it down to 5.8 to 6.
As important are nutrients for a plant, the ability to absorb them is also quite pivotal. The PH level of the plant ascertains its absorption ability. Thus, if the PH levels of a plant go upscale, the absorption rates for nutrients go down. In the same way, low levels suggest over-absorption of nutrients, causing toxicity.
Nutrients Get Affected by Fluctuating PH Levels

Nutrients in plants are classified into two broad categories – Micronutrients and Macronutrients. The former suggests nutrients that are required in small quantities whereas the latter suggests higher quantities required.
All nutrients have their electric charges that remain unmovable. However, plants often swap them with equal amounts of positive and negative ions of hydrogen.
Thus, the cations and the anions are at equilibrium when the PH is neutral. When it increases, the number of anions increase. And when it goes down, cations outrun the number of anions
There are plenty of nutrients required by a plant, in varying quantities. These nutrients have traits that get affected by fluctuations in the PH levels. For illustration, we shall observe two major nutrients – Calcium and Iron.
Both above-mentioned nutrients belong to the categories of Macronutrients and Micronutrients respectively.
In the case of Calcium, a high PH is observed by whitish deposits on the reservoir walls. This is because insoluble salts precipitate out of the nutrient solutions in such situations. On the other hand, a low one indicates calcium deficiency. Leaves often cup and burnt tips are seen.
With Iron, a fluctuation between the positive and negative ions is commonly seen. When any plant experiences a high PH level, it also goes through iron deficiency. This is because iron turns ferric because of its reaction with hydrogen ions which makes it unavailable to plants. This may cause yellow or pale leaves in young plants.
Buffering Capability

The ability of a solution to withstand a change in PH is its buffering capability. Where this most often takes place in hydroponics, because people use hard water in the majority and it contains carbonates.
In buffering the water at a high PH, these are extremely fine. In some cases, a consistently high one might be too stubborn to be lowered. Here, it is likely that the solution’s carbonates improve its buffering ability.
It is recommended to get the water supply checked and probably use an RO (Reverse Osmosis) to sort out minerals if a high level of carbonate exists.
Why Do PH Levels Fluctuate?
In a hydroponics system, multiple factors may cause PH levels to alter. The solution becomes more concentrated when plants consume the nutrients. This happens when the volume of the nutrient solution drops below one gallon.
As a result, PH levels are constantly going up and down. Therefore, monitoring mineral nutrient levels, holding the reservoirs full, and routinely checking it in the reservoir is necessary.
In a hydroponics system, both natural and inorganic materials can influence PH levels. Sediments and other inorganic rising media function as a buffer and trigger levels to increase. In a natural world, soil functions in a fashion similar to a barrier.
Keeping a Check on the PH

The first step towards maintaining the PH levels in a hydroponics system is to monitor it at regular intervals. There exist many ways to do so, for a complete guide just visit this website. Meanwhile, here are a few things:
1. Litmus Paper Test
This one is quite famous and the most trusted since school times. Also, these are pretty cost-effective. The PH level of any solution can be assessed by dipping a strip of the litmus paper in the solution. One gets to know the acidity by comparing the strip to a PH colour chart. Thus, this is an affordable, amateur and speedy way to check its levels.
2. Liquid Test Kits
These comprise sensitive colour changing dyes that need to be dropped in the solution to check its acidity. These are trusted more than litmus paper as they overrun in terms of accuracy. The downside is they are a bit more costly than traditional litmus paper. It is the most popular testing method among the Hydroponics system hobbyists.
3. Electronic PH Testing Meter
This method demonstrates the result very efficiently and effectively as the most high-tech form of PH testing. On the device’s panel, the PH number will be printed. So there’s no need to equate it with the colour map.
There is a selection of varying shapes and costs of electronic PH metres. By far the most widely used is a PH pen. After some time, the metre can drift and display inaccurate results. For its life extension, it needs constant maintenance and diligent handling.
The Real Deal – Maintaining the PH Levels

There are various methods to maintain PH levels in a hydroponics system. There exist organic as well as machine-aided techniques.
To maintain the right PH levels, commercially prepared “PH up” and “PH down” devices are accessible.
Automatic PH controls cost more than products with PH up or down, but the PH is maintained at stable levels. In recirculating systems, this choice functions best to avoid PH disturbances that emerge as plants grow.
The buffering effect of the high mineral levels can cause high PH levels if the water is hard. A powerful and reasonably inexpensive technique for reducing water hardness is a reverse osmosis process.
One can also regulate the PH level of their solutions by adding a little amount of Phosphoric acid to lower it. On the other hand, adding a small amount of potassium hydroxide will raise the PH levels in a hydroponics system.
As a home remedy, people even use acetic acid (white vinegar) and citric acid to bring down PH levels. However, these are not long term solutions.
Final Words
Maintaining the PH for a Hydroponics system is small but significant. To ensure healthy produce, one ought to take necessary measures.



